Welcome to
(844) 549-0883 Xarelto Lawsuit Updates
By (844) 549-0883 Xarelto Lawsuit Updates
Stroke Bleed On Brain
Parent category
Stroke Bleed On Brain
Stroke Bleed On Brain: How Long Will A Xarelto Lawsuit Take?
Call and file a claim today - (844) 549-0883
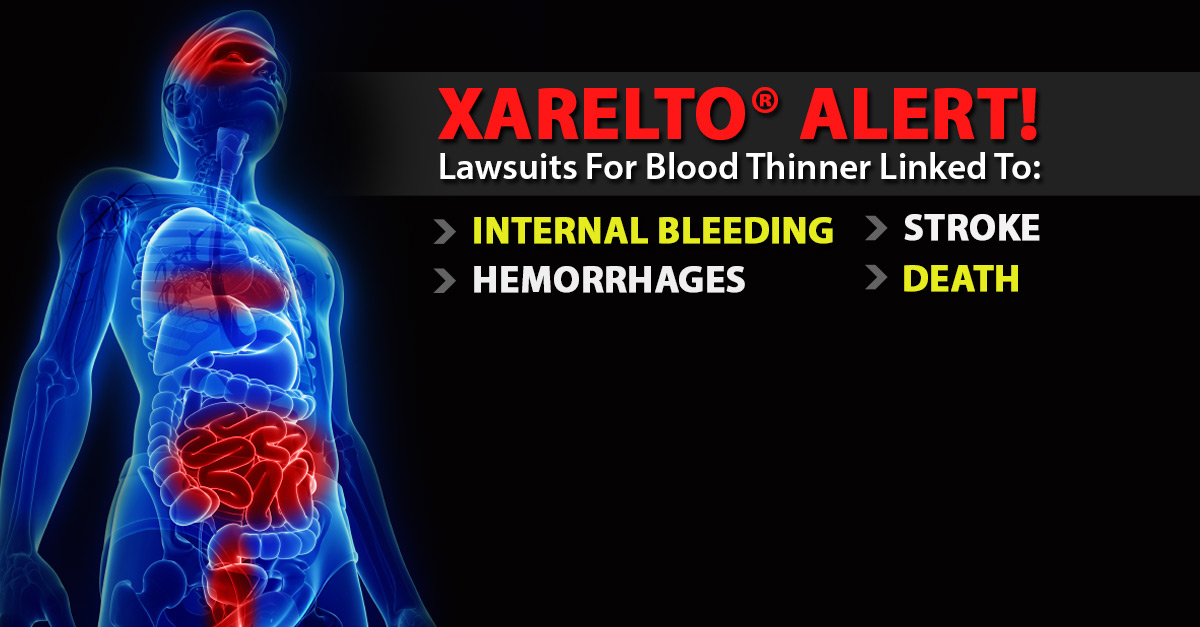
One of the many questions we get from our clients on Stroke Bleed On Brain and during intake is a) whether there have been any Xarelto settlements to date and b) how long will a Xarelto settlement take. The first lawsuits filed alleging that Xarelto caused internal bleeding, which resulted in serious injury or death, were filed against the manufacturers of the blood thinner in early 2014. These bleeding events include:
- gastrointestinal bleeding
- pulmonary embolism
- brain hemorrhage
- epidural hematoma
- rectal bleeding
On April 21, 2015, Mr. Joseph Osborne, recently commented on the pending Xarelto litigation:
We really are in the infancy of this litigation. It will take several years for the litigation to progress through the judicial system whereby we believe Bayer AG and Johnson & Johnson will likely have to pay out substantial compensation to those injured or families who have lost a loved one due to the side effects of Xarelto.
Stroke Bleed On Brain - Xarelto Settlement And Litigation Process
Filing Of Lawsuits
The first phase will be the filing of Xarelto complaints in various jurisdictions around the country, in both state and federal court. These complaints will layout the basis of the Xarelto lawsuit and will identify the damage, pain and suffering endured by the plaintiff.
Based on the numbers of prescriptions of Xarelto issued, and the incidence of an adverse event from the scientific studies, we believe there will be thousands of lawsuits filed alleging that Xarelto caused an adverse event such as an uncontrollable internal bleed which lead to severe injuries or death.
Formation Of MDL
At the end of 2014, the Xarelto litigation advanced to the next phase where all federal filed Xarelto lawsuits were centralized under an MDL or Multidistrict Litigation group at the Eastern District of Louisiana under presiding District Judge Eldon Fallon. Once a mass tort has several filings, either party can petition the court to consolidate the claims in an MDL which will expedite the cases and conserve judicial resources. This mechanism enables lawyers to focus on organization of cases, and discovery in preparation for initial trials and settlement talks. This process will take at least one year.
The First Xarelto Trials
Upon completion of discovery, designated plaintiffs counsel will identify several cases from the pool of cases that are ready for trial. These cases are known as “bellweather cases”. These trials are not expected to take place before 2016. Each trial will take at least one month, and the verdict of each trial will be an indication of the settlement value of a Xarelto lawsuit.
Xarelto Settlement
Settlement negotiations will likely take place in 2016 at the earliest where the defendants will likely settle all the federal cases in one mass settlement. This is similar to how the Pradaxa blood thinner litigation played out where they settled 4,000 cases for $650 million. If no settlement is reached, trials will continue to be scheduled.
Determining Factors At Xarelto Settlements
From meeting our clients and speaking with several victims, we understand the hardship and pain endured by the people who suffered an adverse event. The settlement negotiations will specifically focus on the factors that a jury would take into account what a jury will take into account at trial. These key factors include:
- what injury was suffered as a result of taking Xarelto?
- how long did the injury last?
- what were the short term and long term effects of the injury on the plaintiff?
- what future care might be needed?
- in the event of death, what financial compensation will no longer be available to dependents?
- in the event of disability, what economic loss will be suffered in the future because of an inability to work?
- in a wrongful death xarelto case, to what extent will loss of companionship exist because of the loss of a loved one?
- what medical expenses were incurred in the past?
- what medical expense will be incurred in the future?
- what burial or funeral expenses were incurred?
Xarelto News & Updates Timeline - Stroke Bleed On Brain
The anticoagulant drug Xarelto represents a new class of blood thinning medications meant to replace Coumadin and warfarin. With claims of fewer side effects and easier prescribing restrictions for patients, the joint effort from Bayer and Johnson & Johnson has already surpassed competitors Eliquis and Pradaxa with over $1 billion in sales.
However, the advent of these next-generation blood thinners hasn’t been all positive. Experts have weighed in with warnings that they might result in a lot more severe internal bleeding risks than warfarin given that there isn’t however an antidote for severe hemorrhaging.
Consumer watchdog groups tracking adverse occasion reports for the FDA have noted thousands of patient complications, and a number of hundred deaths filed with the agency.
And now, with the number of lawsuits alleging internal bleeding hospitalization from Xarelto on the rise around the country, lawyers have asked that cases be moved to a special drug injury federal court (read more).
Xarelto Lawsuit Status Update: January 31, 2017
Reviewing Expert Testimony
The federal court Xarelto cases continue to move forward. Numerous expert reports have been exchanged by the parties and depositions of the experts have been taken.
At this point, Xarelto is in the legal briefing period, where both parties test the legal validity of each other’s expert reports. A hearing has been set in March of 2017 by the Honorable Eldon J. Fallon to hear argument about the legal validity of the Xarelto experts’ reports and depositions.
After that hearing, Judge Fallon will issue an opinion about the experts. The opinion will frame the Xarelto litigation for the parties. After the opinion is issued, if the experts are found to be sound, the cases are expected to proceed to trial.
In the Philadelphia County Xarelto litigation, the parties have picked 24 cases for trial work up and the depositions are ongoing. After the depositions are finished, a smaller pool of cases will be picked to be worked up further for trial.
Here, we are working vigorously to proceed in both Federal Court and in Philadelphia County to pursue our client’s claims.
Xarelto Lawsuit Status Update: January 3, 2017
14,000 Xarelto Lawsuits Pending, Four Bellwether Trials Set for Spring 2017
There are now almost 14,000 cases pending in the Xarelto MDL. US District Judge Eldon Fallon of the Eastern District of Louisiana will hear the first case in March 2017 from plaintiff Joseph Boudreaux. Mr. Boudreaux was hospitalized in early 2014 shortly after he started using Xarelto to help control his atrial fibrillation. He required multiple blood transfusions and has accused Janssen and Bayer of misrepresenting the safety profile of Xarelto.
Other Xarelto bellwether trials are scheduled for April 24 and May 30. More Xarelto lawsuits are being filed in courts around the country – learn more about Xarelto lawsuits here.
Xarelto Lawsuit Status Update: September 27, 2016
Deposition of Defendants’ Witnesses Almost Complete – Defendants Say They Will Not Settle Cases
The Xarelto case continues to move at a fast pace. The depositions of Defendants’ witnesses are almost completed and expert reports are due by mid November 2016. The parties will then take depositions of the experts.
The Defendants repeatedly say that the case will not settle, so the parties are expecting to try a lot of cases. In Federal Court, the first four trials have been delayed a little but there should be four cases tried to verdict by early Summer, 2017.
In Philadelphia, 10 trials are set for trial beginning at the end of Summer 2017, with cases being tried every two weeks for basically the rest of 2017.
Depositions of plaintiffs, medical providers and sales representatives continues in federal court and those depositions should be completed by October 3, 2016.
A very important date in the litigation is February 10, 2017, when the court will hear arguments about whether each parties experts should be allowed to testify. Basically, an expert is allowed to testify if the expert’s opinion is based on generally accepted science and the expert’s testimony will be helpful to the jury.
There are approximately 5,000 Xarelto cases filed in state and federal court. There are more cases filed every week. Fortunately, there are two very experienced judges managing the ligation. Lawyers from Nastlaw have devoted countless hours to working in the federal and state litigation. At Nastlaw, we firmly believe in working in both courts so our clients receive the best representation possible.
Xarelto Lawsuit Status Update: August 17, 2016
17 Xarelto Trials Scheduled for 2017
The Xarelto litigation is on track for 17 trials on 2017. This is an unusually fast pace for a large mass tort. In Xarelto, 17 cases will be tried in under three years from the date the first Xarelto lawsuit was filed.
The fast pace of Xarelto is due to a unique combination of factors:
First, the vast majority of the cases on Stroke Bleed On Brain are being managed by two experienced and effective judges. In Federal Court, the Honorable Eldon Fallon is managing the litigation. In the past, Judge Fallon has managed several large MDLs, including Vioxx. In State Court, the Honorable Arnold New is managing the litigation. Judge New has managed numerous mass torts, including Yaz.
Second, Judge New and Judge Fallon have appointed lawyers to manage the litigation that have devoted their careers to mass torts. At NastLaw, Judge Fallon has appointed Dianne Nast to the Plaintiffs Steering Committee. Judge Fallon has also appointed Daniel Gallucci as a state liaison to the Federal Litigation. Judge New has appointed Daniel Gallucci as a co-liaison, which is the functional equivalent of co-lead counsel in the Xarelto Litigation.
This combination of experienced judges and experienced lawyers, hopefully, will result in injured plaintiffs getting their day in court with a speed that is virtually unheard of in mass tort litigation.
First Four Xarelto Trials Identified
On August 17, 2016, the the Honorable Eldon Fallon, who manages the Federal Xarelto litigation entered a case management order identifying the four cases that will be tried in federal court.
Judge Fallon identified the following cases for trial:
- Trial 1: The matter of Joseph Boudreaux, Case No. 2:14-cv-02720, in the Eastern District of Louisiana.
- Trial 2: The Matter of Joseph Orr, Jr., Case No. 2:15-cv-03708, in the Eastern District of Louisiana.
- Trial 3: The Matter of Dora Mingo, Case No. 2:15-cv-03469, in Mississippi.
- Trial 4: The Matter of James Henry, individually and as executor for the Estate of William Henry, Case No. 2:15-cv-00224, in Texas.
Xarelto Lawsuit Status Update: August 4, 2016
On August 4, 2016, the Honorable Eldon Fallon, the Federal Judge managing most of the Xarelto lawsuits in the United States held a hearing to discuss trial dates, coordinating the Federal litigation with the Xarelto litigation in Pennsylvania, and other issues.
Judge Fallon, in essence, ordered that the parties pick specific cases for trial by August 12, 2016. These cases will be tried in 2017.
Judge Fallon also requested the parties to work with the Xarelto litigation in Pennsylvania. There are over 800 Xarelto cases in Pennsylvania and the Honorable Arnold New manages those cases.
Judge New, in Pennsylvania, has directed the parties get 10 cases ready for trial starting in the Summer of 2017.
In total, Judge Fallon and Judge New manage over 95 percent of the Xarelto cases in the United States.
At Nastlaw, we litigate cases in front of both Judge Fallon and Judge New to make sure our clients receive the benefits of the work being done in both the Xarelto Federal litigation and the Xarelto state litigation.
Nastlaw will continue to update our website about the Xarelto lawsuits to keep you informed of important developments in the litigation.
Xarelto Lawsuit Status Update: July 2016
The Honorable Eldon Fallon, the Judge who is managing the Xarelto Federal litigation, has directed the parties to have cases ready for trial by February 6, 2017. Judge Fallon has set four trials. The first trial is set to take place in the Eastern district of Louisiana on February 6, 2017. Judge Fallon has scheduled three other trials to take place in March, April and May of 2017.
To be able to possess the instances prepared for trial; Judge Fallon has ordered the parties to take up to 50 depositions, to become completed by September 23, 2016. These deposition are of workers from the Defendants who have been involved the improvement, marketing and sales of Xarelto.Additionally, the Defendants have been ordered to produce approximately 50 million documents also relating to the development, marketing and sales of Xarelto.
While the Plaintiffs analyze the documents and take the deposition of Defendants’ witnesses, at the same time, plaintiffs who claim that they were injured by Xarelto and filed lawsuits, are being deposed by the Defendants.
By the end of September 2016, approximately 40 cases will be ready to be tried to a Jury.
Xarelto Lawsuit Status Update: February 2016
The Food and Drug Administration is investigating whether the results of the pivotal clinical trial used to approve Xarelto, known as the ROCKET AF trial, are still valid. This clinical trial compared the effectiveness of Xarelto to Warfarin and showed the two to be roughly equal in effectiveness. However, the trial results have been called into question because the device used to measure the amount of Warfarin in patients’ bodies has been recalled.
In the Xarelto litigation, we are also demanding documents and taking depositions on this issue. The validity of the ROCKET AF trial is a key question to whether Xarelto is safe and effective for use in patients with atrial fibrillation. We will continue to investigate this important safety issue.
Xarelto Lawsuit Information - Stroke Bleed On Brain
Xarelto Lawsuits allege that the manufacturers of Xarelto:
- Failed to fully investigate the safety risks of Xarelto
- Failed to provide adequate warnings about the true safety risks associated with the use of Xarelto
- Failed to adequately disclose the increased risk of severe bleeding while taking Xarelto
- Failed to adequately disclose that there is no antidote to stop bleeding from Xarelto
- Over promoted the drug to consumers and healthcare professionals, leading to an explosion in Xarelto sales and putting millions of users at risk
Question:
What is the Latest Status Update for the Xarelto Litigation Over Bleeds, Deaths?
Answer:
A growing number of product liability lawsuits continue to be filed in courts throughout the country on behalf of individuals who allege that side effects of Xarelto caused severe bleeds and other problems, such as:
- Gastrointestinal Bleeding
- Brain Bleeding
- Hemorrhagic Stroke
- Death
The first complaints were filed in early 2014, and by mid-2015 the number of claims involved in the Xarelto litigation has already reached 2,000. However, our Xarelto injury lawyers believe that several thousand additional cases are yet to be filed, and the litigation remains relatively in the early stages.
The lawyers at Saiontz & Kirk, P.A. are continuing to review potential Stroke Bleed On Brain for individuals and family members of Xarelto users who have been hospitalized or suffered severe injury as a result of bleeding events that doctors could not stop due to a lack of a Xarelto reversal agent.
In December 2014, the U.S. Judicial Panel on Multidistrict Litigation established consolidated proceedings for all Xarelto injury cases, transferring all complaints filed throughout the federal court system to U.S. District Judge Eldon Fallon in the Eastern District of Louisiana.
As element of the coordinated pretrial proceedings, Judge Fallon has indicated that a tiny group of representative cases inside the litigation will be prepared for early trial dates to help gauge how juries are most likely to respond to specific evidence and testimony which is likely to be repeated throughout a number of other circumstances.Throughout most of 2015, it is expected that the litigation for Xarelto will be focused on the coordination of discovery from the drug maker, including the review of millions of internal documents surrounding the research, development and marketing of the anticoagulant.
The first Xarelto trial dates are not expected to begin until August 2016. While the outcomes will not be binding on other claims, they may influence eventual negotiations to reach Xarelto settlements.
Allegations Raised in Xarelto Bleeding Litigation
All of the complaints in the Xarelto injury litigation involve similar allegations that Bayer Healthcare and Johnson & Johnson failed to adequately warn consumers or the medical community about the risk of serious and uncontrollable bleeding events associated with the blood thinner.
Xarelto was released in November 2011, as one of a number of new blood thinners designed to prevent strokes due to atrial fibrillation. The drug was launched about a year after its main competitor, Pradaxa.
Drug makers hoped that Xarelto would replace Coumadin (warfarin) as the go-to drug for atrial fibrillation stroke prevention, and as a wide-spectrum use blood thinner.
However, all blood thinners contain the threat of bleeding events. To counteract that danger, warfarin has an antidote, vitamin K, which operates as a reversal agent, allowing doctors to easily cease and treat warfarin bleeding events in most cases.
That is not the case with Xarelto. It was released with no reversal agent and doctors were given no strategy to control Xarelto bleeds. Some make-shift treatments have been used and developed, but barring a future Xarelto update, there is no approved Xarelto reversal agent.
Centralized Management of Xarelto Lawsuits
The U.S. JPML decided to establish a federal Xarelto MDL (multidistrict litigation) in December 2014, which is designed to reduce duplicative discover, avoid conflicting pretrial rulings and to serve the convenience of the parties, witnesses and courts.
Similar consolidation occurred in Pradaxa lawsuits, which was the first member of this new generation of blood thinners to hit the market. Following several years of litigation, the makers of that anticoagulant agreed to pay a $680 million settlement to resolve about 4,000 Pradaxa bleeding claims.
For individuals who suspect they may have a potential claim, it is important to review your case with a lawyer as soon as possible, as a Xarelto statute of limitations will limit the amount of time available in each case to join the litigation.
Thinking About Filing A Xarelto Lawsuit?
If you or a loved one suffered an injury or death because of an adverse event you believe was caused by Xarelto or Stroke Bleed On Brain, then feel free to contact our compassionate, experienced lawyers today. We will evaluate your claim and advise you of your legal rights. We are helping families and individuals all across the United States who believe they have been wronged by the manufacturers of Xarelto. Call us today. The call is free and there is no fee for our services until we recover financial compensation for you.
Xarelto Rivaroxaban 20 Mg Warfarin Class Action Lawsuit
More Xarelto Lawsuit Lawyers Information:
Xarelto Bleeding
Xarelto Substitute
Xarelto Rivaroxaban
Xarelto Side Effects And Uncontrolled Bleeding
Xarelto Inr
When To Take Xarelto
Xarelto Lawsuit Claims
Antidote For Xarelto
Lawsuit Involving Xarelto
What Pain Reliever Can I Take With Xarelto


